- 阻害剤
- 研究分野別
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- 化合物ライブラリー
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleck.co.jp to customize your library.
You could select:
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-II
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Covalent Inhibitor Library
- FDA-approved Anticancer Drug LibraryNew
- Highly Selective Inhibitor Library
- HTS Library for Drug Discovery
- Metabolism Compound Library
- 抗体
- 新製品
- お問い合わせ
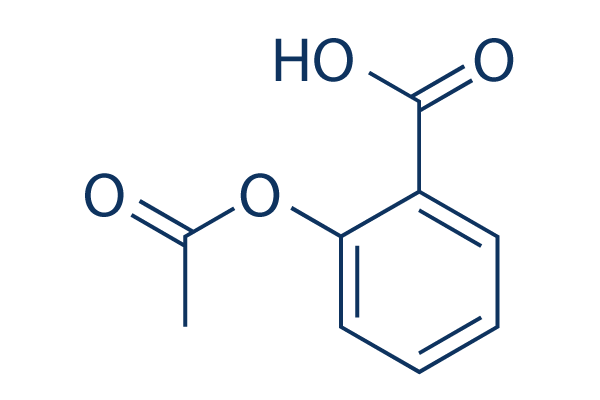
Aspirin
別名:NSC 27223, Acetylsalicylic acid, ASA
Aspirin (NSC 27223, Acetylsalicylic acid, ASA) is a salicylate, and irreversible COX1 and COX2 inhibitor, used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. Aspirin induces autophagy and stimulates mitophagy.
CAS No. 50-78-2
文献中Selleckの製品使用例(25)
製品安全説明書
Aspirin関連製品
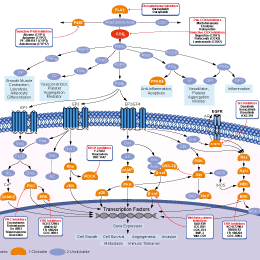
シグナル伝達経路
COX阻害剤の選択性比較
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | 活性情報 | PMID |
|---|---|---|---|---|---|
| PANC1 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PANC1 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 |
| PC3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PC3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 |
| SKBR3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human SKBR3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 |
| 他の多くの細胞株試験データをご覧になる場合はこちらをクリックして下さい | |||||
生物活性
| 製品説明 | Aspirin (NSC 27223, Acetylsalicylic acid, ASA) is a salicylate, and irreversible COX1 and COX2 inhibitor, used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. Aspirin induces autophagy and stimulates mitophagy. | ||
|---|---|---|---|
| Targets |
|
| In Vitro | ||||
| In vitro | Aspirin inhibits the activation of NF-kappa B, thus prevents the degradation of the NF-kappa B inhibitor, I kappa B, and therefore NF-kappa B is retained in the cytosol. Aspirin also inhibits NF-kappa B-dependent transcription from the Ig kappa enhancer and the human immunodeficiency virus (HIV) long terminal repeat (LTR) in transfected T cells. [1] Aspirin and salicylate are mediated in part by their specific inhibition of IKK-beta, thereby preventing activation by NF-kappaB of genes involved in the pathogenesis of the inflammatory response. [2] Aspirin is protective against neurotoxicity elicited by the excitatory amino acid glutamate in rat primary neuronal cultures and hippocampal slices. [3] Aspirin triggers transcellular biosynthesis of a previously unrecognized class of eicosanoidsduring coincubations of human umbilical vein endothelial cells (HUVEC) and neutrophils [polymorphonuclear leukocytes (PMN)]. Aspirin evokes a unique class of eicosanoids formed by acetylated PGHS-2 and 5-lipoxygenase interactions. [4] Aspirin treatment inhibits the phosphorylation of IRS-1 at Ser307 as well as the phosphorylation of JNK, c-Jun, and degradation of IkappaBalpha in 3T3-L1 and Hep G2 cells treated with tumor necrosis factor (TNF)-alpha. Aspirin treatment inhibits phosphorylation of Akt and the mammalian target of rapamycin (but not extracellular regulated kinase or PKCzeta) in response to TNF-alpha. Aspirin rescues insulin-induced glucose uptake in 3T3-L1 adipocytes pretreated with TNF-alpha. [5] | |||
|---|---|---|---|---|
| 実験結果図 | Methods | Biomarkers | 結果図 | PMID |
| Western blot | MCL-1 p-AKT / AKT / p-ERK / ERK p-AMPKα / AMPKα / p-ACC / ACC SHH / SMO / GLI1 / Bcl-2 / Foxm1 |
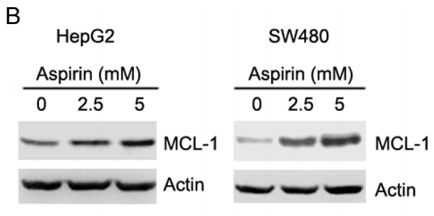
|
26918349 | |
| Growth inhibition assay | Cell proliferation Cell viability |
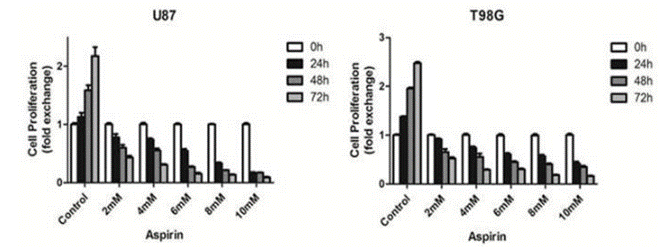
|
28446712 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05960864 | Not yet recruiting | Ankylosing Spondylitis (AS) / Radiographic Axial SpA (r-axSpA)|Non-radiographic Axial Spondyloarthritis (Nr-axSpA)|Axial Psoriatic Arthritis (axPsA)|Acute Anterior Uveitis (AAU)|Crohn Disease (CD)|Ulcerative Colitis (UC)|Reactive Arthritis (ReA) |
Southwest Hospital China |
September 2024 | -- |
| NCT05868525 | Recruiting | Blunt Cerebrovascular Injury |
Loma Linda University |
July 2024 | Phase 4 |
| NCT06197009 | Not yet recruiting | Axial Spondyloarthritis |
Sinocelltech Ltd. |
July 1 2024 | Phase 2 |
| NCT06378398 | Not yet recruiting | Colorectal Neoplasia |
University of Michigan Rogel Cancer Center|National Cancer Institute (NCI) |
May 2024 | Early Phase 1 |
化学情報
| 分子量 | 180.16 | 化学式 | C9H8O4 |
| CAS No. | 50-78-2 | SDF | Download Aspirin SDFをダウンロードする |
| Smiles | CC(=O)OC1=CC=CC=C1C(=O)O | ||
| 保管 | |||
|
In vitro |
DMSO : 36 mg/mL ( (199.82 mM); 吸湿したDMSOは溶解度を減少させます。新しいDMSOをご使用ください。) Ethanol : 36 mg/mL Water : Insoluble |
モル濃度計算器 |
実験計算
投与溶液組成計算機(クリア溶液)
ステップ1:実験データを入力してください。(実験操作によるロスを考慮し、動物数を1匹分多くして計算・調製することを推奨します)
mg/kg
g
μL
匹
ステップ2:投与溶媒の組成を入力してください。(ロット毎に適した溶解組成が異なる場合があります。詳細については弊社までお問い合わせください)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
計算結果:
投与溶媒濃度: mg/ml;
DMSOストック溶液調製方法: mg 試薬を μL DMSOに溶解する(濃度 mg/mL, 注:濃度が当該ロットのDMSO溶解度を超える場合はご連絡ください。 )
投与溶媒調製方法:Take μL DMSOストック溶液に μL PEG300,を加え、完全溶解後μL Tween 80,を加えて完全溶解させた後 μL ddH2O,を加え完全に溶解させます。
投与溶媒調製方法:μL DMSOストック溶液に μL Corn oil,を加え、完全溶解。
注意:1.ストック溶液に沈殿、混濁などがないことをご確認ください;
2.順番通りに溶剤を加えてください。次のステップに進む前に溶液に沈殿、混濁などがないことを確認してから加えてください。ボルテックス、ソニケーション、水浴加熱など物理的な方法で溶解を早めることは可能です。
技術サポート
ストックの作り方、阻害剤の保管方法、細胞実験や動物実験の際に注意すべき点など、製品を取扱う時に問い合わせが多かった質問に対しては取扱説明書でお答えしています。
他に質問がある場合は、お気軽にお問い合わせください。
* 必須
Tags: Aspirinを買う | Aspirin ic50 | Aspirin供給者 | Aspirinを購入する | Aspirin費用 | Aspirin生産者 | オーダーAspirin | Aspirin化学構造 | Aspirin分子量 | Aspirin代理店