|
受注:045-509-1970 |
技術サポート:[email protected] 平日9:00〜18:00 1営業日以内にご連絡を差し上げます |
化学情報
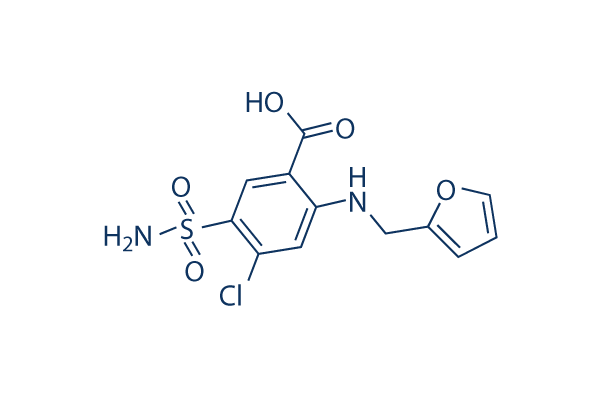
|
Synonyms | NSC 269420 | Storage (From the date of receipt) |
3 years -20°C powder 1 years -80°C in solvent |
| 化学式 | C12H11ClN2O5S |
|||
| 分子量 | 330.74 | CAS No. | 54-31-9 | |
| Solubility (25°C)* | 体外 | DMSO | 66 mg/mL (199.55 mM) | |
| Ethanol | 20 mg/mL (60.47 mM) | |||
| Water | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
||||
溶剤液(一定の濃度)を調合する
生物活性
| 製品説明 | Furosemide is a potent NKCC2 (Na-K-2Cl symporter) inhibitor, used in the treatment of congestive heart failure and edema. |
|---|---|
| in vitro | Furosemide reversibly alters the responses to tones and clicks of the chinchilla basilar membrane in hair cells, causing response-magnitude reductions that are largest (up to 61 dB, averaging 25-30 dB) at low stimulus intensities at the characteristic frequency (CF) and small or nonexistent at high intensities and at frequencies far removed from CF. Furosemide also induces response-phase lags that are largest at low stimulus intensities (averaging 77 degrees) and are confined to frequencies close to CF. [1] Furosemide concentration- and time-dependently increases the formation of nitric oxide andprostacyclin. Furosemide leads to an enhanced release of kinins into the supernatant of the cells. [2] Furosemide reversibly suppresses low Ca2+-induced epileptiform activity in hippocampus proper and blocks or significantly reduces different types of epileptiform discharges in the low Mg2+ model and the 4-aminopyridine model. [3] Furosemide significantly inhibits cell growth in MKN45 cells, but not in MKN28 cells. Furosemide diminishes cell growth by delaying the G(1)-S phase progression in poorly differentiated gastric adenocarcinoma cells, which show high expression and activity of NKCC, but not in moderately differentiated gastric adenocarcinoma cells with low expression and NKCC activity. [4] |
| in vivo | Furosemide (0.25 to 2 mg/kg), administered 4 hours before exercise, reduces right atrial pressure (RAP) and pulmonary arterial pressure (PAP) during exercise in dose-dependent manner in horse. [5] |
プロトコル(参考用のみ)
| 細胞アッセイ | 細胞株 | Erythrocytes |
|---|---|---|
| 濃度 | 10, 30 and 100 µM | |
| 反応時間 | 45 minutes | |
| 実験の流れ | Cells were treated with indicated concentrations of drug for 45 minutes. |
|
| 動物実験 | 動物モデル | CD-1 male mice |
| 投薬量 | 3 mg/kg | |
| 投与方法 |
参考
Selleckの高級品が、幾つかの出版された研究調査結果(以下を含む)で使われた:
| A novel contrast-induced acute kidney injury mouse model based on low-osmolar contrast medium [ Ren Fail, 2022, 44(1):1345-1355] | PubMed: 35938700 |
| Evidence for Specific Receptor-Mediated Toxicity of Pharmaceuticals in Aquatic Organisms Derived from Acute and Chronic Standard Endpoints [ Environ Toxicol Chem, 2021, 10.1002/etc.5018] | PubMed: 33595135 |
| Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat [Choi SY, et al. Biomed Pharmacother, 2018, 101:145-154] | PubMed: 29482060 |
| Gallic acid improves cardiac dysfunction and fibrosis in pressure overload-induced heart failure [Jin L, et al. Sci Rep, 2018, 8(1):9302] | PubMed: 29915390 |
| Gallic acid attenuates pulmonary fibrosis in a mouse model of transverse aortic contraction-induced heart failure [Jin L, et al. Vascul Pharmacol, 2017, 99:74-82] | PubMed: 29097327 |
長期の保管のために-20°Cの下で製品を保ってください。
人間や獣医の診断であるか治療的な使用のためにでない。
各々の製品のための特定の保管と取扱い情報は、製品データシートの上で示されます。大部分のSelleck製品は、推薦された状況の下で安定です。製品は、推薦された保管温度と異なる温度で、時々出荷されます。長期の保管のために必要とされてそれと異なる温度で、多くの製品は、短期もので安定です。品質を維持するが、夜通しの積荷のために最も経済的な貯蔵状況を用いてあなたの送料を保存する状況の下に、製品が出荷されることを、我々は確実とします。製品の受領と同時に、製品データシートの上で貯蔵推薦に従ってください。
